
Environment Targets & Initiatives
Medium-Term Environmental Action Plan 21-25
Mitsubishi Tanabe Pharma Group views environmental measures as an important management issue and has identified “environment-friendly business” as a materiality that contributes to the SDGs, and has formulated the Medium-Term Environmental Action Plan 21-25, which established six environmental themes, including monitoring indicators, as priority items.
Raising GHG Emission Volume Reduction Targets
The Group has raised its greenhouse gas (GHG) emissions volume reduction targets for the Medium-Term Environmental Action Plan 21-25 based on the significant reductions anticipated in GHG emissions volumes through action plans intended to achieve this under an action plan to reduce these emissions that was decided on in-house for fiscal 2023.
GHG emission volume (Global: Scope 1 + 2)
- FY2025 target: 25% -> 58% reduction compared to FY2019
- FY2030 target: 45% -> 69% reduction compared to FY2019
State of Medium-Term Environmental Action Plan 21-25 Achievement
| Targets | Principal Initiatives and Results in Fiscal 2023 | Environmental SDGs | |
|---|---|---|---|
| Energy conservation and global warming mitigation |
|
|
Goal 7 Goal 13 |
|
|
||
|
|
||
| Reduction of waste, recycling and reuse of resources |
|
Compared to fiscal 2019
|
Goal 12 |
|
|
||
| Effective use of water resources |
|
|
Goal 6 |
| Prevention of environmental pollution |
|
|
Goal 6 Goal 12 |
|
|
||
| Preservation of biodiversity |
|
|
Goal 15 |
| Enhancement of environmental management |
|
|
|
|
|
Material Balance
The figures below show the amount of resources (inputs) directly consumed and the environmental impact (outputs) discharged by our business activities in fiscal 2023.
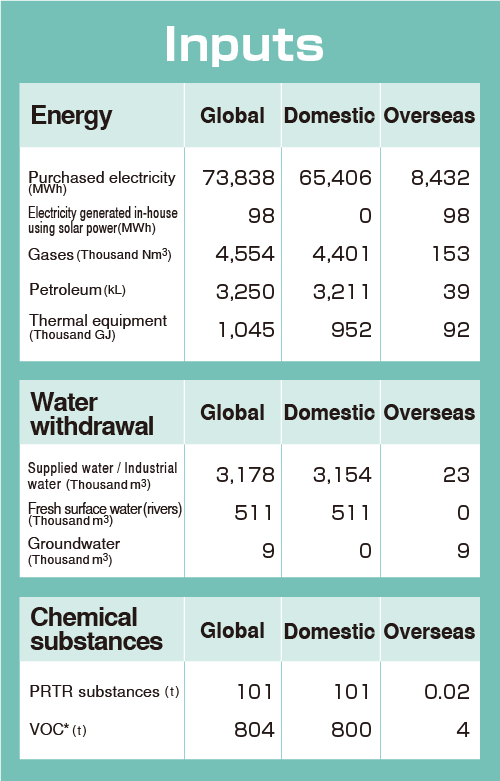
*Excluding PRTR subtances
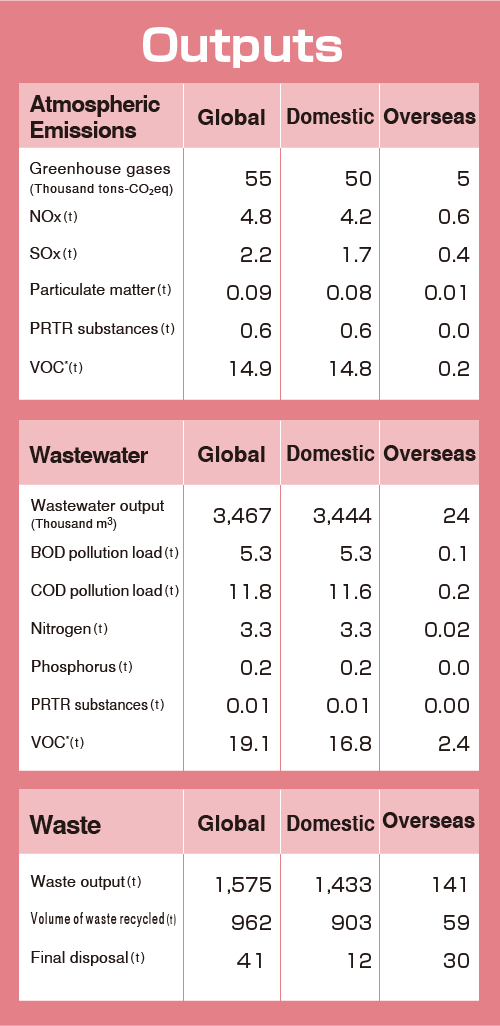
[PDF:168KB]
Participation in Initiatives and Industry Group Activities
The Group participates in the following initiatives and industry group activities to solve social issues related to the environment and continues to be a company that is trusted by society.
Activities of Japan Climate Initiative (JCI)*
In an effort to achieve the decarbonized society required in the Paris Agreement, the Company has participated in the Japan Climate Initiative* since 2021. Additionally, JCI has been sending regular messages to encourage the Japanese Government to achieve the target of limiting global temperature increase to the 1.5°C set out in the Paris Accords, and in fiscal 2023, we expressed our support for the “Overcoming Two Crises with Renewable Energy and Carbon Pricing” message.
*The Japan Climate Initiative (JCI) is a network comprised of various entities (non-government actors) besides the national government that includes companies, municipalities, and NGOs, aiming to achieve a carbon-free society. Companies that are actively working on climate change measures are joining in support of the JCI Declaration which states, “Joining the front line of global trend for decarbonization from Japan.”
Activities of Pharmaceutical Industry Associations
The Company participates as a member of the Environmental Committee of The Federation of Pharmaceutical Manufacturers’ Associations of Japan and contributes to formulating guidelines and action plans for the industry. We also participate in the Carbon Neutral Working Group and are working to achieve the carbon dioxide emissions reduction target based on Japan Business Federation’s (Keidanren’s) requests. Furthermore, we are participating in an environmental issues study group established by the Japan Pharmaceutical Manufacturers Association (JPMA) in fiscal 2022, and are working to address environmental issues as a pharmaceutical industry.
