
Society > Together with Patients and Healthcare Professionals Stable Supply
Stable Supply of Pharmaceuticals
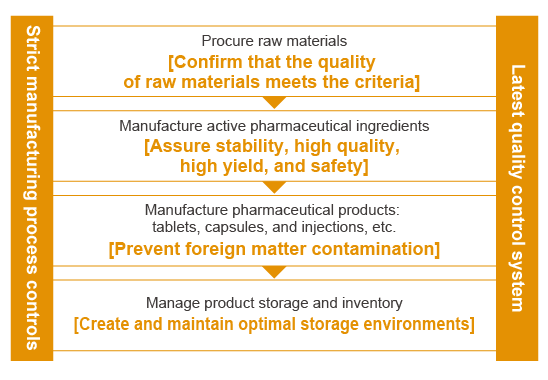
The Mitsubishi Tanabe Pharma Group manufactures and supplies high-quality pharmaceuticals and strictly controls product quality from acceptance testing of raw materials procured in Japan and overseas to the manufacture of GMP-compliant active pharmaceutical ingredients and drug products as well as testing and inspection, thus supplying premium quality products which patients and healthcare professionals can use safely and with peace of mind. As a global research-driven pharmaceutical company, we manufacture pharmaceuticals based on a wide range of technologies and proprietary know-how developed over many years.
To further ensure quality, the Product Supply Division, the CMC Laboratories, and the Global Quality Assurance Department collaborate with the Group’s manufacturing plants to develop production technologies to enhance quality, stabilize supply, and reduce costs from the early development stages of new pharmaceutical products. In addition, the Group’s manufacturing plants (two in Japan and three overseas) together with manufacturing subcontractors are creating a global production system that delivers a stable supply of our products to many people around the world.
In June 2016, we built a new domestic manufacturing facility within the Yoshitomi Plant for solid dosage formulations. This highly productive facility can supply pharmaceuticals in accordance with global quality standards, while further contributing to both the improvement of manufacturing technologies and the reduction of manufacturing costs.
In addition, BIKEN Co., Ltd., a joint venture with the BIKEN Foundation’s vaccine manufacturing business, began operation in September 2017. By strengthening our manufacturing base with the Biken Foundation, we aim to ensure a more stable supply of vaccines. In March 2024, we released a five-in-one vaccine (GOBIK Aqueous Suspension Syringes) that added the antigen component of Haemophilus influenzae type b (Hib) to the existing four-in-one vaccine, contributing to reducing the number of routine vaccinations required.
We had already diversified and decentralized our procurement systems for drug substances and raw materials, and furthermore, ensured those safety stocks in response to the spread of COVID-19 beginning in December 2019, and to the world-wide supply uncertainty that was deepened by the Russian Federation’s invasion of Ukraine in February 2022 and the deteriorating situation in the Middle East. Moreover, we are building reliable partnerships with our suppliers and working to ensure a continuous stable supply.
Manufacturing Systems in Asia Other than Japan
In Asia, we have manufacturing/sales bases in South Korea, Taiwan, and Indonesia, and we provide products that meet the quality standards and market needs in each country.
In Asian markets—especially the ASEAN pharmaceutical market—growth is expected, and to meet this growing demand, we increased production capacity at Mitsubishi Tanabe Pharma Indonesia, an Indonesian subsidiary that produces oral agents for the domestic Indonesian and ASEAN countries, and in 2015, built a new building to manufacture drug preparations with the aim of accommodating versions of PIC/S GMP (Indonesia).*
Mitsubishi Tanabe Pharma Korea, a local subsidiary in South Korea, manufactures high quality injections and other pharmaceuticals as a PIC/S GMP level manufacturing facility, and supplies South Korea, as well as Europe, Japan, China, and parts of Mongolia. In addition, Taiwan Tanabe Seiyaku, a local subsidiary in Taiwan, has cleared PIC/S GMP certification and manufactures high-quality oral and external preparations. Among these, sugar-coated tablets are also exported to Japan.
In the future, we will continue to expand business in Asia, a growth market. By providing a stable supply of high-quality products, we will contribute to people around the world who want to live a healthy and prosperous life and fulfill our corporate social responsibility.
*PIC/S: Abbreviation for Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme

Mitsubishi Tanabe Pharma Korea

Taiwan Tanabe Seiyaku

pharmaceutical production building,
Mitsubishi Tanabe Pharma Indonesia
Managing Distribution to Ensure Stable Supplies
As a pharmaceutical company, Mitsubishi Tanabe Pharma steadily and reliably provides high-quality pharmaceuticals, when they are needed and to the patients who need them. We have built a supply system that can provide a stable supply of drugs to patients, even in the event of a disaster or other unexpected situation.
Initiatives at the Distribution Center
Supply system
We ship drugs to customers through a dual-base supply system comprising the New East Japan Distribution Center (Kuki City, Saitama Prefecture) and the New West Japan Distribution Center (Kobe City, Hyogo Prefecture). To reduce a variety of risks that could adversely affect a stable supply, both centers have earthquake isolation systems, in-house power generators, and redundant installations of important equipment. In this way, they are designed to be able to maintain a supply of drugs, even during major disasters and pandemics. For example, even if one distribution center becomes inoperable at any time, the other distribution center will be able to provide a continued supply of pharmaceuticals to customers. Even if the system server is hit by a disaster, maintaining a stable supply is our top priority, so we have built a system that instantly shifts to an alternate server at another location.
- Beginning joint transportation in domestic logistics
- From January 2023, the Company began joint transportation with two other companies in compliance with the GDP guidelines for the transport of medical pharmaceuticals in domestic logistics. We have jointly established standards for managing transport, and transport products efficiently by ensuring quality through temperature control along transportation routes from each company’s logistics centers to pharmaceutical wholesalers.
In transporting the products of three companies together, the Company has established collaborative systems for the resolution of issues such as reducing the number of vehicles and lowering transport costs and CO2 emissions. We have been able to maintain stable operations since the initiative started, and this initiative has gained a solid reputation for addressing issues in the transportation industry, which is experiencing a driver shortage, not only from customers but also within the industry.
Incoming/Outgoing shipments and inventory control procedures
The distribution centers employ an inventory control system that accurately and carefully monitors incoming and outgoing shipments and inventory control procedures in lot units. The introduction of the inventory control system enables the Company to appropriately store and control pharmaceuticals in a variety of categories, such as by pharmaceutical characteristics and storage temperatures. In addition, in response to data received from higher level systems, we can rapidly conduct operations without mistakes.
Training
We periodically conduct training for the employees who use the distribution center facilities and systems. In this way, we seek to enhance the skills of each employee and to reduce human error. At the same time, by heightening awareness of pharmaceutical distribution extending all the way to the patient, we are working to build a system that can maintain a secure, safe, and stable supply of drugs.
Quality Control in the Distribution Process
Mitsubishi Tanabe Pharma distribution centers take a rigorous approach to quality control in the distribution process. This attention to detail helps ensure that pharmaceuticals are as high in quality when they reach patients as they are when manufactured under the strict GMP of the Company’s production plants.
Meeting GDP
The Company complies with the structural facility requirements under the Pharmaceuticals and Medical Devices Law (The Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices) of Japan and other relevant regulations as well as various operational requirements. In addition, it has constructed a system that meets the Japanese version of Good Distribution Practice (GDP) guidelines. In light of the characteristics of the pharmaceuticals that we handle, we have developed distribution policies, procedure manuals, and facilities for “quality assurance (especially temperature control),” “proper control of the distribution process,” and “preventing contamination by counterfeit medicines and their distribution,” which are shown in the guidelines. We strictly observe these policies and manuals in the conduct of our business in order to maintain distribution quality in terms of both the physical and operational aspects.
Handling of cold storage products
In particular, for cold storage products, which require rigorous temperature control, in addition to periodic temperature validation and thermometer calibration in cold warehouses, the Company has emergency response measures in place, including a process that provides information when abnormal or emergency conditions are detected and in-house power generators that can be used when electricity is interrupted. In this way, the Company has designed a system that can maintain appropriate temperature management, 24 hours a day, seven days a week.
Creating a transportation system
Mitsubishi Tanabe Pharma designed its entire transportation system with the focus on supplying high-quality pharmaceuticals. Pharmaceutical products are shipped from the distribution centers via contracted transport companies that comply with pre-determined qualifications. With an understanding of the characteristics and importance of the pharmaceuticals that they are carrying, these companies strictly supervise the transport of this cargo, utilizing facilities and vehicles specifically designed for loading and unloading pharmaceuticals. The Company works to maintain quality during the distribution process by carrying out regular inspections of its subcontracting transport companies, as well as using a comprehensive distribution method with precise temperature control monitoring and special insulated boxes for packing the products.
Preventing contamination by counterfeit medicines and their distribution
Counterfeit medicines could cause damage to the health of many random patients, which is a major health and hygiene issue. To deliver quality medicines to patients, the distribution center has created a system to prevent contamination by counterfeit medicines and the distribution of medicines of questionable quality, including counterfeit medicines.
We regularly verify and record that all customers have received proper authorization to buy medicines (shipped to customers).
To strictly manage medicines, we restrict the people who can enter the storeroom of the distribution center and prescribe the method of entering. In addition, when storing pharmaceuticals, we confirm that the received medicines are correct and have no visible damage.
If counterfeit medicines or medicines of questionable quality are found, we have created a system to immediately suspend sales and transport, and to segregate, and report them to government agencies.
Together with Patients and Healthcare Professionals
- Research & Development
- Stable Supply
- Manufacturing Pharmaceuticals that are Secure, Safe, and Convenient to Use
- Information Provision
- Drug Safety / Quality Assurance
- Solving Issues Related to Improving Access to Healthcare
Together with Employees
- Human Resources Development
- Promoting Diversity & Inclusion
- Work-Style Innovation
- Occupational Health and Safety
- Health and Productivity Management
