
Society > Together with Patients and Healthcare Professionals Drug Safety / Quality Assurance
Quality Assurance System of Drugs
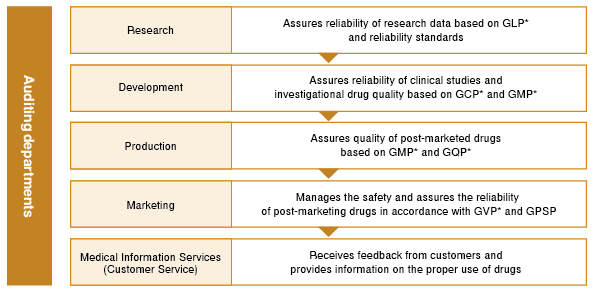
In April 2020, we established the Quality & Vigilance (QV) Division to function as the global head of quality and safety management for products. The QV Division has the following functions.
Primary Functions of the QV Division
- Creation of a mechanism and system for the stable supply of high-quality products
- Audits to ensure the reliability of each operation from research and development to post-marketing
- Collection and analysis of safety information of products and products under development, and the reporting and dissemination of that information
- Formulation and promotion of safety information surveillance policies for post-market products
To ensure that our pharmaceuticals can be used by healthcare professionals and patients with peace of mind, the QV Division strives to maintain and improve our system of reliability assurance by complying not only with “The Law on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices,” but also various laws and regulations including GLP, GCP, GMP, GQP, GDP, GVP, and GPSP.
In May 2017, we obtained approval for edaravone as a treatment agent for amyotrophic lateral sclerosis (ALS) in the U.S. Subsequently, we also obtained approval in Switzerland, Canada, and other countries, and have accelerated product rollout in regions where we have not established our own sales system.* More than ever, we are providing products that comply with the regulations of each country while collaborating with the quality and safety departments in each country. Always mindful of differences in medical environments, we provide patients around the world with products that they can use with peace of mind.
The Group will continue to ensure the quality, effectiveness, and safety of pharmaceutical products by complying with laws and regulations and maintaining and improving its quality assurance system.
*A system of direct sales as well as sales by licensed overseas companies. The establishment of a direct-sales system enables independent operation as a pharmaceutical company.
- *GLP (Good Laboratory Practice)
Standards for conducting preclinical trials on pharmaceutical safety. - *GCP (Good Clinical Practice)
Standards for conducting clinical trials of pharmaceuticals. - *GMP (Good Manufacturing Practice)
Standards for manufacturing and quality control of pharmaceutical and quasi-pharmaceuticals. - *GQP (Good Quality Practice)
Standards for quality control of pharmaceuticals, quasi-pharmaceuticals, cosmetics, and medical devices. - *GVP (Good Vigilance Practice)
Standards for post-manufacturing and marketing safety management of pharmaceuticals, quasi-pharmaceuticals, cosmetics, medical devices, and regenerative medical products. - *GPSP (Good Post-marketing Study Practice)
Standards for conducting post-marketing surveillance and studies of pharmaceuticals.
New Drug Safety Management
After the launch of a new drug, adverse drug reactions that were not discovered in clinical trials are sometimes reported. We quickly collect that information, analyze it, and provide feedback to the medical front lines. We are moving forward with proactive safety management activities that incorporate new safety measures. We believe that these activities help prevent the expansion of adverse drug reactions from new drugs and promote appropriate usage on the medical front lines.
Furthermore, overseas use involves different medical environments from that in Japan, and it will therefore be necessary to exercise caution in safety management.
For example, edaravone, which was discovered by the Company, was approved in Japan in 2001 as a treatment for the acute ischemic stroke and has been in use for more than 20 years. Since 2015, edaravone has been used in Japan as well as overseas in countries such as the U.S. to treat ALS.
The abundant safety information that we have accumulated has given us valuable experience in promoting its proper use. Making full use of that experience and taking into account the overseas regulatory and medical environments, we will work to collect and provide safety information to foster proper use and to contribute to improvement in the quality of life of ALS patients.
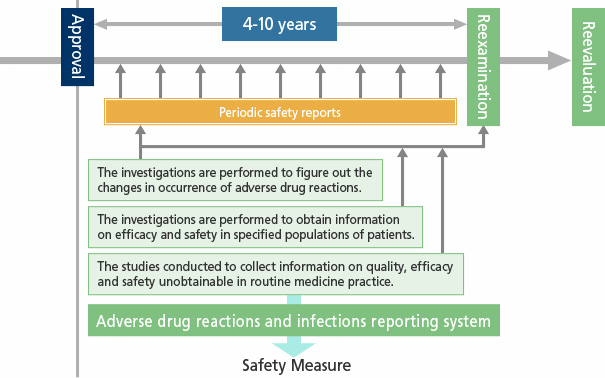
Post-Marketing Surveillance in Japan
After the regulatory authority approves the manufacturing and marketing of a drug based on the results of nonclinical and clinical studies, we begin selling the drug. Clinical studies are conducted with the number of subjects that are required to scientifically verify the efficacy and safety of the new drugs. However, the conditions for participation in clinical studies (age, medical history, concomitant medications, etc.) are not necessarily the same as the post-marketing conditions of use.
Therefore, the Company starts to collect safety information under actual conditions of use at medical sites as soon as drugs are launched, and we also conduct various post-marketing surveillance. Through the surveillance, we aggregate safety information regarding the drugs that have been actually prescribed to patients, we monitor the safety and efficacy of drugs, and the information that is obtained in the surveillance is quickly and accurately provided to the regulatory authorities and healthcare professionals. In this way, we are working to support the proper use of drugs.
Quality of Products
Our policy* is to contribute to the health and well-being of people around the world through the stable supply of high quality, reliable products which are manufactured under a world-class quality system. On that basis, we are strictly observing the ministerial ordinance on GMP (regulations regarding pharmaceutical manufacturing control and quality control) and on GQP (regulations regarding pharmaceutical quality control). Patient safety is the first priority of every employee, and we are implementing initiatives targeting quality assurance with a focus not only on results but also on processes. Through management, supervision, and guidance of manufacturing sites in Japan and overseas, we are working to improve the quality of the products that we provide to the market.
Furthermore, we are working to ensure the quality of pharmaceuticals in accordance with a division notification from the Ministry of Health, Labour and Welfare dated June 1, 2016, regarding the carrying out of production based on the certificate of approval for manufacture and sales.
Above all, to ensure patient safety and prevent disadvantage, any problems found in the safety, effectiveness, quality, labeling, and other aspects of a product should be promptly reported to the regulatory agency and information provided to the medical institution, and a system should be in place to recall the product.
In fiscal 2023, we conducted two (class II) voluntary recalls in Japan, but no related health problems were reported.
In addition to the quality of pharmaceuticals that can be used with peace of mind by patients, one of our important missions is to supply pharmaceuticals when patients need them. Therefore, from fiscal 2021, we set the number of product recalls as a monitoring indicator of our materiality and ensure a stable supply of pharmaceuticals.
Quality assurance initiatives
- Strengthen collaboration with manufacturing plants and reinforce checking systems, and regularly confirm actual manufacturing practices and the certificate of approval
- Enforce measures to prevent recurrence by rectifying and improving any defects based on our own checks and investigations at manufacturing plants
Pharmaceutical Safety Training
We are working to accumulate and pass on knowledge, and raise awareness related to pharmaceutical safety for management and all employees.
In fiscal 2023, we also conducted educational training on pharmaceutical safety management and drug-induced suffering. As people who work at a pharmaceutical company, the training improved the risk sensitivity of each of us, so that we always act with high ethical standards with the patient’s health and safety as our highest priority.
Together with Patients and Healthcare Professionals
- Research & Development
- Stable Supply
- Manufacturing Pharmaceuticals that are Secure, Safe, and Convenient to Use
- Information Provision
- Drug Safety / Quality Assurance
- Solving Issues Related to Improving Access to Healthcare
Together with Employees
- Human Resources Development
- Promoting Diversity & Inclusion
- Work-Style Innovation
- Occupational Health and Safety
- Health and Productivity Management
