
Society > Together with Patients and Healthcare Professionals Solving Issues Related to Improving Access to Healthcare
There are many intractable diseases in the world for which no cure has been found, as well as many difficult-to-cure diseases. Notably, research and development of therapeutic agents for infectious diseases such as malaria, tuberculosis, and NTDs, which are prevalent in developing countries, is not progressing due to unpromising marketability. Furthermore, inadequate medical systems, poverty, and disasters in developing countries prevent them from receiving needed medicines and medical services.
To address these issues of access to healthcare, the Group will leverage its strengths in drug discovery, and work in partnership with NPOs/NGOs, industry groups, and others based on our MISSION of “Creating hope for all facing illness.”
Intractable Disease Initiatives
We have created treatment options for intractable diseases such as inflammatory bowel disease and multiple sclerosis.
Providing new options for diseases for which there has been no cure is our MISSION itself. We seek to realize precision medicine for diseases for which unmet medical needs remain, especially in the central nervous system and immunoinflammatory areas. In addition, we will contribute to improving the quality of life of patients and their families by providing solutions based on therapeutic medicine from prevention to prognosis.
For the materiality monitoring indicator, we have designated a new “Development pipeline quantity for rare and intractable diseases,” and have disclosed the pertinent results from fiscal 2021.
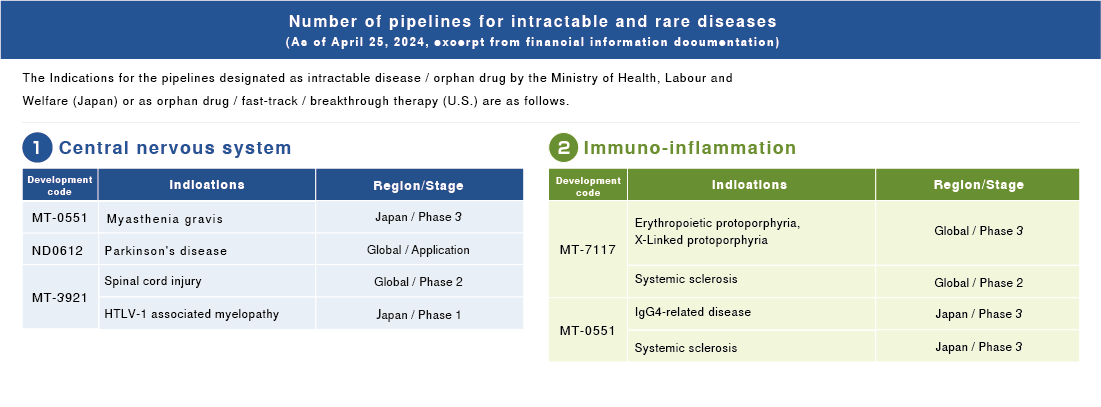
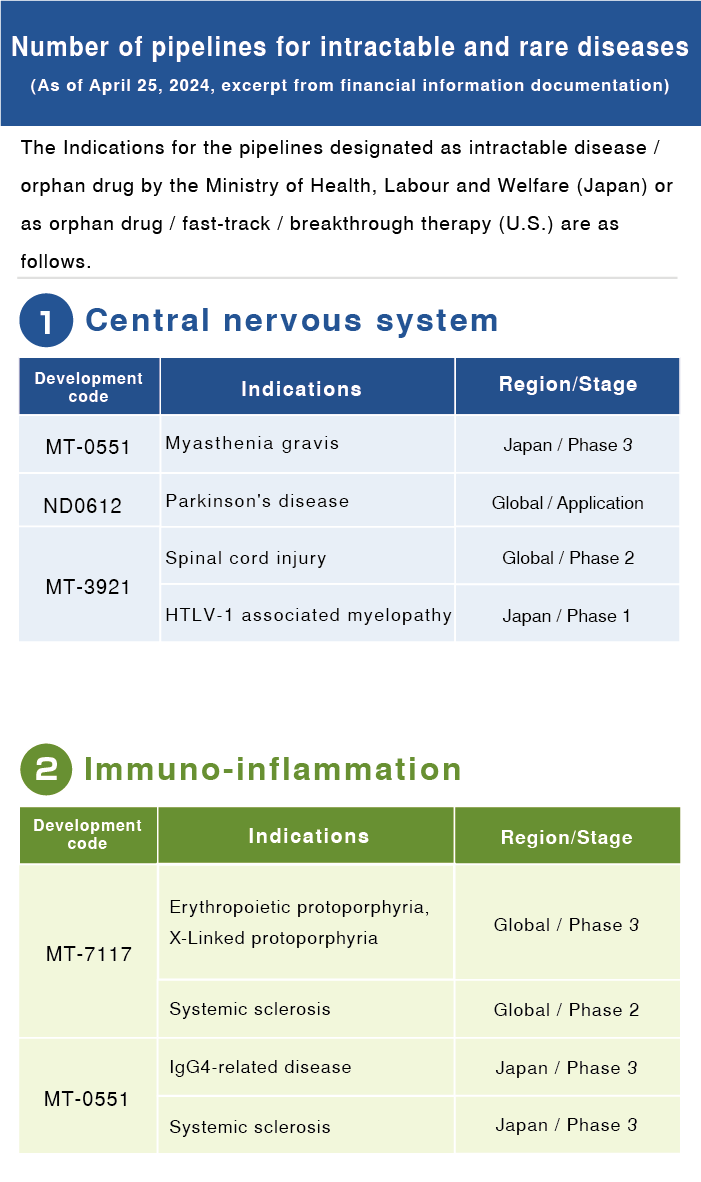
Dersimelagon is being developed as a new treatment option for erythropoietic protoporphyria and X-Linked protoporphyria, which causes painful skin symptoms when exposed to sunlight. In 2021, we began clinical trials of Dersimelagon as a treatment for systemic sclerosis, which is a rare disease and characterized by hardening of the skin and internal organs.
Going forward, we will work on research and development to attain our MISSION and contribute to the realization of a healthy and sustainable society by offering a promising option to a large number of patients around the world who are struggling with intractable diseases, as well as to their families.
Global Health
Controlling infectious diseases that are prevalent in developing countries leads to improved living conditions for people and the overcoming of poverty, creating a virtuous cycle not only in developing countries but throughout the world. As a drug discovery company, we believe that leveraging our strengths in drug discovery is the best way to contribute, and we are conducting research on therapeutic drugs for infectious diseases through the GHIT Fund. We also provide various support through collaborative research efforts with non-profit organizations aimed at creating new antimicrobial medications for use against drug-resistant bacteria, and by paying attention to intellectual property in developing countries and donating to NPOs and NGOs.
Participation in the Global Health Innovative Technology Fund
The Global Health Innovative Technology Fund (GHIT Fund), is Japan's first public-private partnership to promote the creation of innovative treatment agents for infectious diseases that affect people in developing countries, such as malaria, tuberculosis, and neglected tropical diseases (NTDs). We support the GHIT Fund’s objective of contributing to global health, and financially support the fund.
Meanwhile, with funding from the GHIT Foundation, we are researching with our partners infectious disease treatments that are widespread in developing countries.
In particular, the eradication of malaria and NTDs presented below is listed as one of the 169 targets linked to the 17 goals of the United Nations’ Sustainable Development Goals (SDGs). Improving medical access is also a materiality for our company, so we will continue to actively promote it.
Joint research with Medicines for Malaria Venture (MMV)
Malaria is one of the three most serious infectious diseases, killing approximately 600,000 people each year. Utilizing funding from the GHIT Fund, we collaborated with MMV (Medicines for Malaria Venture), to start a joint antimalarial drug research in December 2014, which featured a discovery screening program that was succeeded in identifying promising hit compounds. In addition, we promoted joint research and from one of these compounds, we acquired two lead compounds as new anti-malaria drug candidates, and moved to the lead optimization stage in April 2019. Moreover, in October 2023, we acquired funding for further research from GHIT Fund, and with the University of Georgia as an additional partner, are continuing collaborative research to create preclinical candidates.
Joint research with Drugs for Neglected Diseases initiative (DNDi)
WHO defines 21 diseases as NTD (Neglected Tropical Diseases) that humanity must control. In September 2019, we launched a collaboration supported by GHIT Fund for a discovery screening program with DNDi (Drugs for Neglected Diseases initiative), which is dedicated to developing drugs for these diseases, and acquired a hit compound for one such neglected disease, Chagas disease. In April 2021, DNDi and we started a Hit to Lead (HL) study for Chagas disease to create lead compounds and succeeded in generating a lead compound with promising efficacy in a preclinical Chagas model. From April 2024, we acquired funding for further research from GHIT Fund and began lead optimization research. Additionally, in fall 2023, Mitsubishi Tanabe Pharma and DNDi were jointly awarded “DNDi 2023 Project of the Year in Pre-Clinical Research” for the results of the joint research.
DNDi Projects of the Year
Every year DNDi offers awards to teams and partners that have achieved significant results in projects conducting preclinical and clinical research. The DNDi Scientific Advisory Committee makes nominations from among more than 40 projects in DNDi’s R&D portfolio, and two of those nominated are selected for awards by Executive Board members from DNDi.
Joint research with GARDP (Global Antibiotic Research and Development Partnership)
Headquartered in Switzerland, the non-profit Global Antibiotic Research and Development Partnership (‘GARDP”) develops new medications for drug-resistant bacterial infections that pose a grave danger to our health. It was established in 2016 by the World Health Organization (WHO) and DNDi (the Drugs for Neglected Diseases initiative) to ensure that everyone who needs antimicrobial drugs has access to effective, affordable treatment.
In November 2022, Mitsubishi Tanabe Pharma concluded a contract with GARDP to offer its screening compound library with the goal of developing new antimicrobial drugs for use against drug-resistant bacteria. This screening resulted in the successful acquisition of several types of hit compounds, and we are currently evaluating the potential for further joint research. Through these initiatives, we support GARDP efforts to address serious bacterial infections caused by drug-resistant bacteria.
Patents in Countries Where Access to Healthcare Is Difficult
Mitsubishi Tanabe Pharma Group has established a policy on intellectual property that forms the basis for providing new healthcare opportunities in order to appropriately protect and make effective use of its globally competitive intellectual property. On the other hand, in countries where serious economic problems make access to healthcare difficult, we need to consider enforcing our patent rights. The Group contributes to healthcare access in economically deprived areas around the world. Therefore, as a general rule, we do not enforce our patent rights in the Least Developed Countries (LDCs) stipulated by the United Nations.
Please see “Protection of Intellectual Property Rights” for details.
Other Support
| Support provided | Description of initiatives | Countries targeted |
|---|---|---|
| Providing vaccines and school meals to children in developing countries | Group employees have participated in vaccine support activities for children in developing countries, which are conducted by the NPO Japan Committee, Vaccines for the World's Children (JCV). This is an international contribution activity wherein donated used books and other items are sold and that amount is donated to JCV and used to deliver vaccines to children in developing countries. Furthermore, at the employee cafeterias, we participate in TABLE FOR TWO (TFT), in which one meal is provided to a child in a developing country for each meal ordered by an employee from our healthy menu. This is a support program conducted by the NPO TABLE FOR TWO International. School meals that are provided through donations are expected not only to help solve hunger among children but also lead to gains in the children’s fundamental strength and help prevent disease. We are actively promoting these initiatives to help raise employee awareness. | Myanmar, Laos, Uganda, Rwanda, etc. |
| Child palliative care in developing countries | In the hope of providing palliative care services equally to all children, Mitsubishi Tanabe Pharma Indonesia donates and provides pharmaceuticals to the NGO Rachel House, a pioneer in palliative care in Indonesia. Through this activity, we help children suffering from serious illnesses in areas outside Jakarta where medical care is unavailable. | Indonesia |
Please see “Contributions to Medical Care and Welfare” for details about these initiatives.
Support for the Kenya Research Station (Nairobi), Institute of Tropical Medicine, Nagasaki University
In developing countries, where medical institutions are not well established, many infants die from the exacerbation of infectious diseases. Through Nagasaki University’s Institute of Tropical Medicine, which conducts collaborative research on rotavirus gastroenteritis, we donated lab equipment to the institute’s Nairobi Research Station laboratory. Further, as part of our development of next-generation human resources, we hired as research interns young Kenyans who aspire to be researchers and engaged them in the work of collecting samples and data at the medical facility and conducting experiments in the lab.
The Nairobi Research Station is located on the premises of the Kenya Medical Research Institute, and is a P2/P3 level facility with molecular biology and pest laboratories. A total of seven administrative teams and 50 staff members including collaborators are on the research team and are active in area fields where epidemiological research is conducted. Although the joint research with MTPC was completed in March 2021, the Nairobi Research Station will continue to study tropical infectious diseases and public health peculiar to Africa to address various medical issues not only in Kenya but also in Sub-Saharan Africa. Moreover, we are developing entry-level human resources through the African Business Education for Youth and the JICA Project for Infectious Disease Control Human Resources Development together with Japan International Cooperation Agency (JICA).


Together with Patients and Healthcare Professionals
- Research & Development
- Stable Supply
- Manufacturing Pharmaceuticals that are Secure, Safe, and Convenient to Use
- Information Provision
- Drug Safety / Quality Assurance
- Solving Issues Related to Improving Access to Healthcare
Together with Employees
- Human Resources Development
- Promoting Diversity & Inclusion
- Work-Style Innovation
- Occupational Health and Safety
- Health and Productivity Management
