
Society > Together with Patients and Healthcare Professionals Information Provision
- MR's Responsibility: Collecting Data and Providing Information to Medical Institutions
- Providing Comprehensive Information through Seminars
- Providing Information for Self-Medication
- Overseas Activities
- Providing Information through Websites
- Providing Comprehensive Information through the Medical Information Center
Pharmaceutical companies must reliably and continuously provide, collect, and communicate all information regarding the quality, efficacy, and safety required for the use of their products to healthcare professionals. Moreover, the information provision activities of pharmaceutical companies are expanding due to the rapid evolution and spread of digital technology. The Mitsubishi Tanabe Pharma Group contributes to healthcare to meet diversifying medical needs. At the same time, we provide appropriate treatment proposals in response to each patient’s condition, and conduct information provision activities for the proper use and dissemination of pharmaceuticals.
MR's Responsibility: Collecting Data and Providing Information to Medical Institutions
The Mitsubishi Tanabe Pharma Group provides information to healthcare professionals around Japan through medical representatives (MRs) and is working to deliver optimal drugs to patients.
The environment around healthcare is changing drastically together with progress in digital technology, and in response to this we are promoting our ZEUS (Zoom on Effective Ultimate System) Digital Marketing Project. In addition to the conventional MR activity of visiting medical institutions, we have also developed hybrid MR activities that make full use of digital channels with online interviews and web content that meet doctors’ needs.
The Important Role of MRs
- Communication of safety information and scientifically based academic information on the proper use of ethical drugs
- Collection of information on drug efficacy and safety that could not be gleaned at the R&D stage, and reporting evaluations based on those results
We have assigned MRs who are specialists in specific disease areas to be responsible for drugs that require a higher level of expertise.
Providing Comprehensive Information through Seminars
By holding disease awareness seminars and events, we provide information to help patients and society deepen their understanding of diseases and lead a better life.
Nikkei Health Seminar 21
In January 2023, “Future ALS Treatment and Care,” Nikkei Health Seminar 21 was held online with our sponsorship, hosted by Nikkei Inc. The day comprised two sections, a lecture by a specialist and a panel discussion.
The lecture given by the specialist was on the theme of “Near-future prospects for ALS.” During the panel, panelists answered questions received in advance from online participants on the theme of “Providing a more reassuring life for ALS patients—Points to be aware of in ALS care,” providing easy-to-understand advice and answers. There were questions from patients with ALS, as well as from doctors who are treating people suffering from it, on subjects such as independent living and the use of ventilators. In answer, there was a great deal of useful information and ideas on offer, such as the possibility of living by themselves if there a range of care services are available.
The content of the seminar was later published in the Nihon Keizai Shimbun’s evening edition.

Online Public Lecture
In June 2023, Mitsubishi Tanabe Pharma Corporation and Eli Lilly Japan K.K., hosted an online public lecture titled “Dealing with diabetes together with your doctor –‘MY target values’ you should know,” in collaboration with the Yomiuri Shimbun. This public lecture was divided into two parts, a lecture and a talk session, with a diabetes specialist and a specialist in behavioral economics appearing as speakers. Diabetes requires long-term treatment, and thus it is important to share your goals with your doctor and work as a team, and the lecturers provided easy-to-understand explanations on what people should know about target values for blood glucose management and gave tips on how to communicate smoothly with your doctor. The content of this seminar was later published in the Yomiuri Shimbun.

- Advocacy Seminar
"Enriching the lives of people with diabetes —What healthcare professionals can do to eliminate prejudice and misunderstanding—" -
On World Diabetes Day on November 14, 2023, Mitsubishi Tanabe Pharma held a web-based advocacy*1 seminar together with JADEC (the Japan Association for Diabetes Education and Care) titled, "Enriching the lives of people with diabetes —What healthcare professionals can do to eliminate prejudice and misunderstanding—."
Recently the stigma*2 associated with diabetes has been a topic of discussion. Held for healthcare professionals, this seminar focused on addressing this issue, and was attended by as many as 9,271 people. It was split into two sections—a lecture and a panel discussion—with the lecture featuring a diabetes specialist, a nurse, and a researcher who offered readily comprehensible talks on the stigma that exists in the medical field and offered suggestions on how to deal with it, as well as on the importance of reviewing knowledge of diabetes treatment. Meanwhile, the panel discussion offered a lively debate on what healthcare professionals can do to dispel diabetes-related stigma, taking the perspectives of diabetes patients themselves into account.
Mitsubishi Tanabe Pharma will continue to work to eliminate the stigma of diabetes so that diabetics can lead fulfilling lives and take a proactive stance with regard to their treatment.- *1 Advocacy: Protecting the rights of people in vulnerable positions in society
- *2 Stigma: A symbol of shame or disgrace. In the medical field, “stigma” refers to discrimination or prejudice against patients

Advocacy seminar
“Enriching the lives of people with diabetes —What healthcare professionals can do to eliminate prejudice and misunderstanding—”
Providing Information for Self-Medication
Self-medication means to be “responsible for one’s own health and self-treatment for minor ailments” (WHO definition). In order to promote self-medication, the Company has set up the Hifu no Koto site, which is supervised by specialists such as doctors and pharmacists, and is conducting educational activities.
To promote self-medication in the area of dermatology, Mitsubishi Tanabe Pharma conducts a variety of educational programs through TV commercials and websites to help people suffering from dermatological problems to obtain accurate information and find a treatment as quickly as possible. Of these, the Hifu No Koto site provides information based on the opinions of experts, such as doctors and pharmacists.
In fiscal 2023, we added a “Search by case image” function that allows users to gain a more appropriate understanding of symptoms. We also increased the number of supervising physicians, broadened the scope of the information we provide to cover a wider range of skin problems such as eczema and dermatitis as well as infectious skin diseases such as ringworm and athlete’s foot, and disseminated a lot of new content. In fiscal 2023, more than 10 million people viewed the site.
In the field of rhinitis, “Talion AR” allergic rhinitis medication has been reclassified from a medication requiring guidance to an over-the-counter medication (category 1 over-the-counter medication) that can be purchased over the internet. By continuing to broadcast television advertisements and offering positive messages such as, “Starting this year, you’re going to like spring more,” we have promoted self-medication to hay fever sufferers. Moreover, as with last year, we have continued our efforts to create a pollen calendar that summarizes pollen dispersal forecasts for each area, which we made available on the brand website for this product in order to promote awareness among hay fever sufferers.
Femcare is a line of products and services intended to care for women’s bodies and health. With a name that combines “Feminine” and “Care,” these have attracted attention over the last few years. Since 2010, the Company has also marketed Okinazole L100 (one tablet a day for six days) for the treatment of recurrent vaginal candidiasis, and we are informing people about the ailment on the brand website and letting them know that they can treat this affliction themselves using over-the-counter drugs. Moreover, in June 2023, we began the sale of Okinazole L600, which is effective with just a single dose. We used this opportunity to update our brand site, adding articles overseen by physicians as well as a store locator function, and are working to further promote self-medication as a way to treat recurrent vaginal candidiasis infections.
*Reference: Hifu no Koto site
URL: https://hc.mt-pharma.co.jp/hifunokoto/ (Japanese language only)
Overseas Activities
The Mitsubishi Tanabe Pharma Group has about 430 MRs overseas who provide appropriate usage information through local overseas subsidiaries in order to support the appropriate use of the Company’s pharmaceuticals. In addition to the U.S., these subsidiaries are located in Europe (U.K., Germany, Austria, and Switzerland) and in Asia (South Korea, Taiwan, Singapore, Indonesia, Thailand, and Malaysia). MRs involved in drug information provision activities visit medical institutions and doctors, participate in related academic conferences, exchange opinions with specialists, and provide the latest academic information. In this way, MRs are working each day to be able to contribute to the diagnosis and treatment activities of healthcare professionals.
Activities in the United States
Edaravone was approved as a treatment for ALS in the U.S. in May 2017 and launched in August. It is marketed by Mitsubishi Tanabe Pharma America (MTPA). Additionally, in May 2022, edaravone oral suspension was approved in the U.S. and launched in June of the same year. MTPA also offers support to ALS patients through the JourneyMate Support Program. This program provides information on ALS and its treatment to patients diagnosed with ALS and their families, tailored treatment management for each patient, insurance reimbursement support, as well as offering information from specialist staff in the US (clinical educators) after a prescription of edaravone.
Furthermore, to support patients and families confronting ALS, we actively participate in disease awareness events, hold webinars for patients, and sponsor patient group events.
- Main initiatives
-
Throughout the year, MTPA participates in events that support ALS patients in order to aid patients living with ALS, along with their families. As a national sponsor, we supported United States ALS Association’s Walk to Defeat ALS, and MTPA employees also participate in events throughout the US. Moreover, we proactively hold and support events such as webinars for ALS patients that are intended to provide useful information on ALS, along with a range of events intended to raise awareness of the disease and educate ALS patients, their families and caregivers. We also provide information on ALS through ALS Pathways and the JourneyMate Support Program, allowing those diagnosed with ALS and their families to access necessary information.

Participating in an ALS awareness event
Activities in Asia
In Asia we are working to bring medication for diabetes and for patients with neuropsychiatric disorders in Taiwan, South Korea, and ASEAN nations quickly.
In Taiwan, we have acquired approval for the neuromyelitis optica spectrum disorder (NMOSD) medication inebilizumab.
In the ASEAN region we received approval for the use of an antipsychotic product (cariprazine) in the treatment of bipolar disorder in Malaysia and Indonesia in August 2022 and February 2023, respectively. We also received approval of valbenazine for the use in the treatment of tardive dyskinesia in Singapore in June 2022, and in Indonesia and Thailand in October of the same year, and launched in Singapore in January 2023.
Through these activities we will continue to provide patients in Asia who are fighting diabetes, neuropsychiatric disorders, and other difficulties with promising treatment options.
Providing Information through Websites
Mitsubishi Tanabe Pharma has set up the following health support websites in Japan and around the world.
On these websites, we provide useful information in an easy-to-understand format with illustrations about the symptoms, diagnoses, and treatment of these diseases that helps many people gain a proper understanding of disease, the importance of treatment, and supports the daily lives of patients and their families. We have also prepared leaflets that summarize the information on the health support websites so that healthcare professionals including doctors and pharmacists can present them to patients and their families.
Status of major site updates in fiscal 2023.
- Inflammatory bowel disease (Crohn's disease / Ulcerative colitis)
- At the “SHITTOKU café,” a website providing information for patients with Inflammatory Bowel Disease, we added a new article to the “Tips for Working with IBD” series on the theme of different ways of living and working (2.) In fiscal 2024, we also launched a new website titled “Check your buttocks problems” that is now in operation.
- Rheumatoid arthritis
- In RHEUMA21.info. we updated the video content provided for patients who are using or considering using our products.
- Amyotrophic lateral sclerosis
- Updated content in “ALS Café web” and “ALS Restaurants for traveling the world” on “ALS Station” as information to help ALS patients lead more fulfilling lives.
- Vaccinations
- Posted a “Vaccine Information” article on “Vaccine.net” allowing the latest news of vaccines to be viewed on the web. Additionally, we posted a “Vaccine roadmap for everyone” that illustrates lifelong vaccinations.
- Tardive dyskinesia
- For “Searchlight: Finding and Supporting Together,” we added a new article titled, “Could this be tardive dyskinesia?” as part of the information we provide on tardive dyskinesis symptoms, treatment, and medical expenses.
- Sleep disorders
- “Suimin Net” features a series of articles that examine the basic mechanisms and the mysteries of sleep, and information on the themes of sleep cycles, the body clock, and sleep needs.
In fiscal 2023, about 20.46 million people visited these health support websites.
- Rheumatoid arthritis
- Crohn's disease
- Ulcerative colitis
- Ankylosing spondylitis
- Behcet's disease
- Amyotrophic lateral sclerosis (ALS)
*Launched in Japan and the U.S. - Brain and nerve diseases
- Multiple sclerosis
- Spinocerebellar degeneration and multiple system atrophy
- Liver failure
- Chronic kidney disease
- Sleep disorders
- Neuromyelitis optica
- Tardive dyskinesia
- Vaccines
- Eczema and dermatitis
- The “Medication Information for Patients” website
-
In November 2023, as a way to promote the appropriate use of medication and deepen patients’ knowledge and understanding, we opened our “Medication Information for Patients” website for patients using our ethical drugs. Patients can now use this website to obtain the latest information on our ethical drugs at any time and from any location. Additionally, this website also serves as a tool for healthcare professionals when providing information on medication for their patients.
Medication Information for Patients (Japanese language only)
https://patients.mt-pharma.co.jp/
Providing Comprehensive Information through the Medical Information Center
Mitsubishi Tanabe Pharma has established its own Medical Information Center to respond directly to inquiries from healthcare professionals (physicians, pharmacists, wholesalers, and others) and patients.
In November 2020, we set up a dedicated contact point for patients using our ethical drugs to contact us by telephone, and in October 2021 by inquiry form. We make it clear to patients and their families that this is an open avenue of contact for our business, differentiated from those for healthcare professionals, and are working to make it easier for them to consult with us. In November 2023, we opened our “Medication Information for Patients” website, which offers patients and their families direct access to information on our ethical drugs.
We are working each day to improve our skills so that we identify the true needs behind the inquiries and respond in a way that increases the satisfaction of the people who call. The Medical Information Center receives more than 38,000 inquiries (FY2023 results) a year on a wide range of subjects. It also provides information on the appropriate use of the Company’s products while utilizing basic pharmaceutical information and the in-house Q&A. Valuable information that the center receives from customers about safety, such as drug side effects, and quality is shared internally. In this way, the center helps improve products, enhance reliability and discover future new drugs.
In recent years, the diversification of information sources for healthcare professionals and patients and the development of digital technology has caused a decrease in the number of telephone inquiries, even in the pharmaceutical industry. On the other hand, the amount of information provided by unmanned information channels has increased. We are enhancing the quality and quantity of the product Q&A provided through our website while adding digital channels (AI chatbot and LINE Official Account) that can better meet customer needs, and are endeavoring to allow people to obtain the information they need 24 hours a day, 365 days a year.
Moving forward, the center will respond flexibly to changes in the times and provide appropriate usage information for pharmaceuticals in a reliable, accurate, and prompt manner. In this way, we will work to contribute to the promotion of patient health.
- LINE Official Account “Mitsubishi Tanabe Pharma Medical info”
-
In March 2023, we opened our LINE Official Account “Mitsubishi Tanabe Pharma Medical info” in order to increase convenience for healthcare professionals. This account allows people to access the product information they want quickly and easily using a smartphone, and we will also use it to deliver the latest information on drug supply, revisions to package inserts and other in a timely fashion.
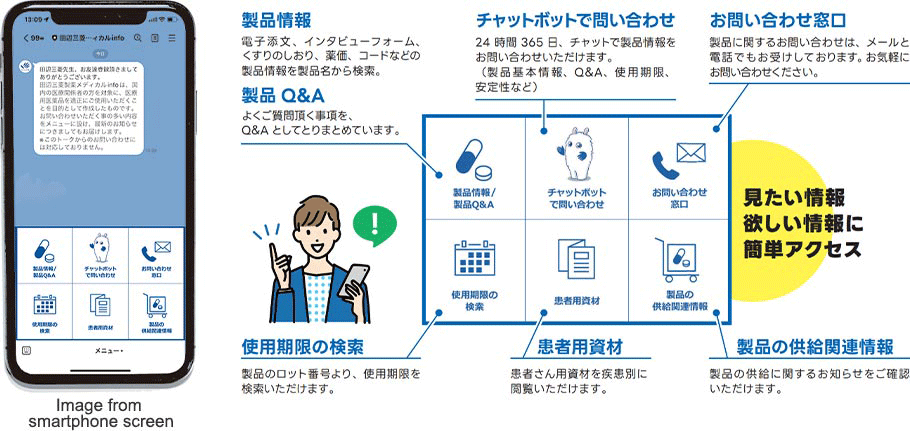
*Note:Mitsubishi Tanabe Pharma Medical info is intended for the proper use of ethical drugs by healthcare professionals in Japan.
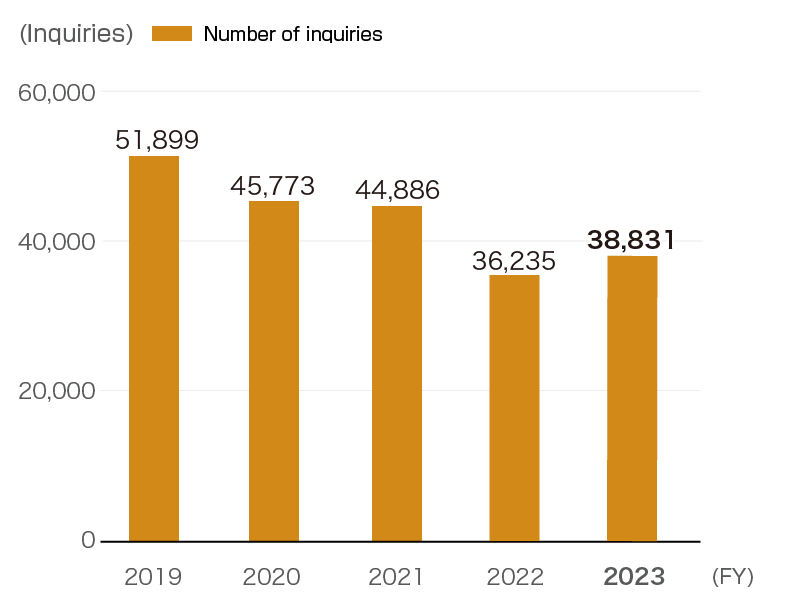
- Note: Decrease in the number of cases due to the transfer of sales of Sun Pharma products.
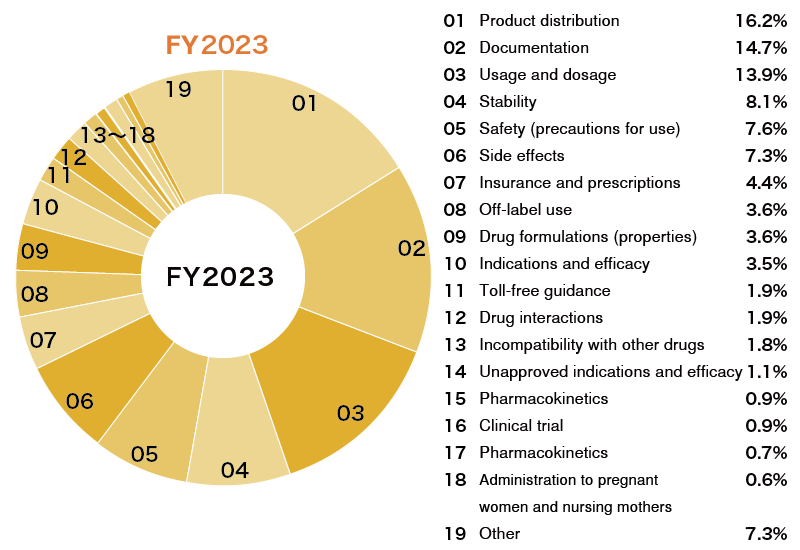
- *Toll-free guidance: Guidance to redirect consumers by providing correct contact information
Other: Inquiries on MR calls, lectures, seminars, doping, and other matters
Together with Patients and Healthcare Professionals
- Research & Development
- Stable Supply
- Manufacturing Pharmaceuticals that are Secure, Safe, and Convenient to Use
- Information Provision
- Drug Safety / Quality Assurance
- Solving Issues Related to Improving Access to Healthcare
Together with Employees
- Human Resources Development
- Promoting Diversity & Inclusion
- Work-Style Innovation
- Occupational Health and Safety
- Health and Productivity Management

