
Society > Together with Patients and Healthcare Professionals Manufacturing Pharmaceuticals that are Secure, Safe, and Convenient to Use
The Company provides pharmaceuticals that can be used in a secure, safe, and convenient manner by patients, healthcare professionals, and others.
This section introduces certain initiatives implemented by the Company to improve drug printing and packaging as well as convenience of use and medication compliance. Moving forward, we will continue working to steadily increase the scope of pharmaceuticals that are subject to these initiatives and to facilitate the provision of pharmaceuticals that are easy to use for patients and healthcare professionals.
Global Vigilance/Safety Policy
Measures to Make Drugs Easier to Use
Developing New Dosage Forms (Reducing the Burden of Taking Medicine)
Up until now it was necessary for ALS patients to go to the hospital or to be hospitalized to receive treatment, which would be administered intravenously using painful needles. To offer ALS patients easy access to medication, in 2018, we began development of an oral suspension of edaravone as an additional form of dosage, with sales beginning in the United States in June 2022. As of March 2023, it has been used to treat approximately 10,000 patients, winning broad praise from the ALS community for eliminating the need to use needles and reducing the burden of home treatment.
In Japan, we obtained marketing approval of the “Edaravone Oral Suspension” for ALS medication in December 2022, with sales beginning in April 2023. Based on edaravone medications, we will continue our efforts to improve the quality of life of ALS patients around the world.
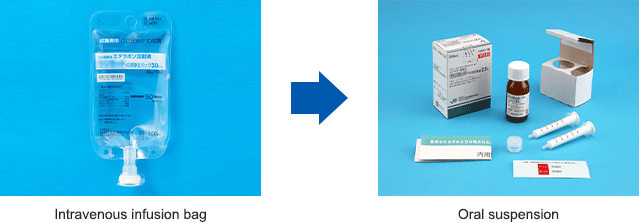
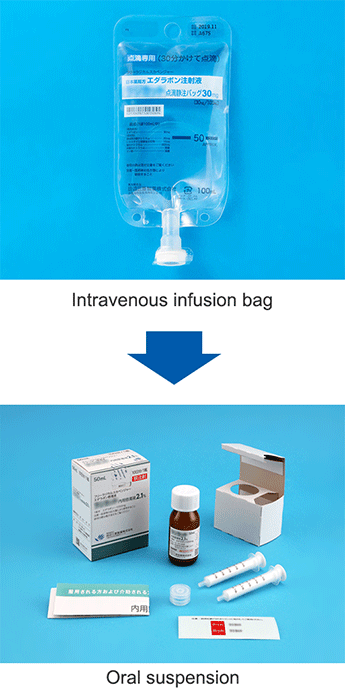
Devising Formulations (Reducing the Burden of Taking Medicine)
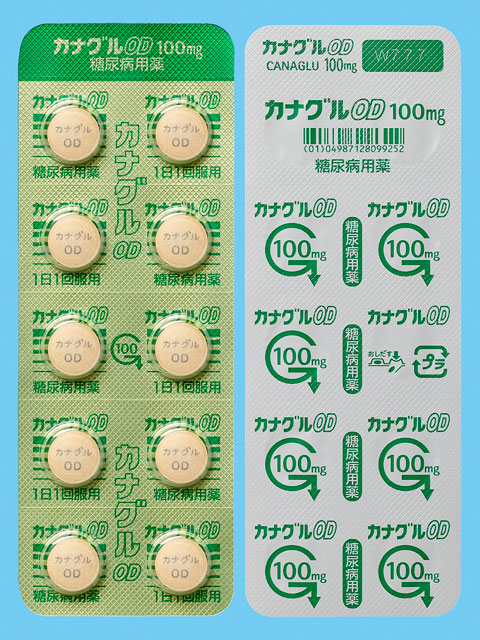
In March 2024, we obtained marketing approval for the OD (orally disintegrating*) tablets for an SGLT2 inhibitor that is used as a treatment for stage 2 diabetes, and launched this product in May. This type of medication dissolves easily in small amounts of water, such as saliva in the mouth, making it easy to swallow. Moreover, it can be taken with or without water, allowing people to use it anywhere, which we think will make it more convenient and will help people continue with their medication.
To help improve the satisfaction that people with type 2 diabetes who require ongoing care have regarding their treatment, in June 2021, we released a selective DPP-4 inhibitor in OD tablet form, and in February 2024, applied for an additional dosage form of OD tablets for the combination drug of selective DPP-4 inhibitor and SGLT2 inhibitor.
*Because orally disintegrating tablets disintegrate in tens of seconds due to saliva or a small amount of water on the tongue, they are also useful for the average person as well as the elderly who cannot swallow tablets easily and those with water intake restrictions.
Source: Pharmaceuticals and Medical Devices Agency
https://www.pmda.go.jp/safety/consultation-for-patients/on-drugs/qa/0002.html
Using Packaging Initiatives to Provide Explanations of How to Take Medicines
Some drugs are difficult to take because of the dosage form. To help patients to take their drugs correctly, we print a QR code on the drug packaging and make it simple for them to view a video that explains in an easy-to-understand manner the basic way to take the drug and necessary precautions. The video is played if a smartphone or other devices are used to read the QR code* printed on the packaging. The objective of this initiative, which is an industry first, is to be useful in such situations as when patients receive compliance instruction at pharmacies and when patients take their drugs.
*“QR Code” is a trademark of Denso Wave Incorporated.
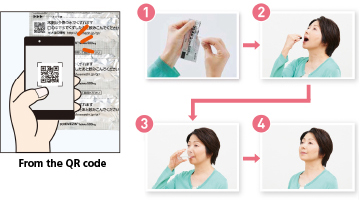
When the QR code is read, a movie is played that explains how to take the quick-disintegrating tablet for chronic renal failure.
The quick-disintegrating tablet does not increase medication volume due to its unique formulation technology and quickly disintegrates with a small amount of water and reduces diffusion into the oral cavity. It is therefore expected to improve the medication compliance of patients who have difficulty taking capsules and fine granules.
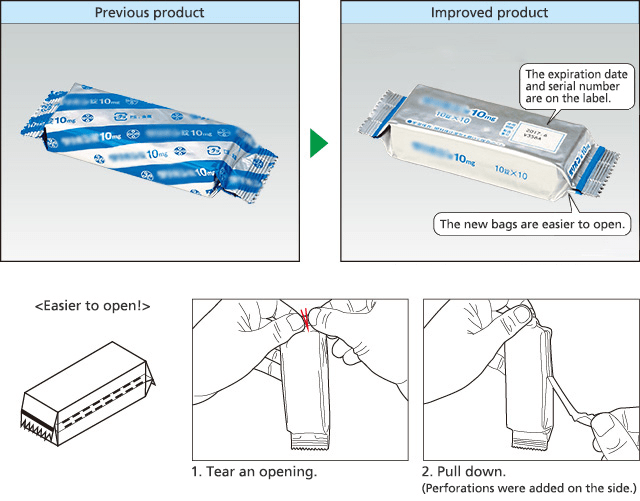
Initiatives with Aluminum Bags (Easier to Open and Easier to Take Out the Product)
We are also working actively to improve the ease of use of pharmaceuticals. Medical institutions provided feedback that the aluminum bags containing the pharmaceutical packaging sheets (PTP sheets) were difficult to open and it was not easy to take out the product. In response, we worked in cooperation with the material manufacturer to develop aluminum bags that are easy to open and make it easy to take out the product. These improved aluminum bags received the Pharmaceutical and Medical Packaging Award at the Japan Packaging Contest 2016 (sponsored by the Japan Packaging Institute).
Measures to Prevent Medical Errors
Printing the Product Name on Both Sides of Tablets
As one measure to prevent medical errors, we print the product name in Japanese on both sides of tablets for such combination drugs as the Selective DPP-4 Inhibitor and SGLT2 Inhibitor, which are treatment agents for type 2 diabetes mellitus. This measure, which replaces the identification code, is expected to help prevent mistakes with tablets during the medicine picking process and to increase efficiency in drug dispensing, as well as to help prevent administration errors by patients.
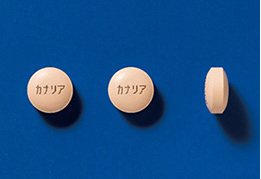
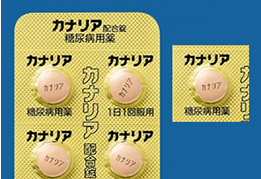
Labeling of Packaging Sheets (Press-through-Package (PTP) Sheets)
In order to help prevent medication errors, some of the Company’s products include the product name and ingredients on every pocket of the packaging sheet (PTP sheet). This enables patients to confirm the product name and content, even when single pockets are removed from the rest of the sheet when the drugs are dispensed. We are also striving to further increase visibility. To that end, we are taking steps to implement designs that are easy to see, such as increasing the size of characters and enhancing color schemes.
Initiatives to Reduce Environmental Impact
Use of PTP Sheets Made from Environmentally Friendly Biomass Plastic
Some of our products utilize PTP sheets made from environmentally friendly biomass plastic. This PTP is a product of the Mitsubishi Chemical Corporation, a member of the same Mitsubishi Chemical Group (MCG Group), and allows carbon dioxide emissions to be reduced by 30-70% in comparison with PTP sheets made from petroleum-derived plastic.*
- *Data source: Mitsubishi Chemical Corporation. For PTP sheets with similar permeability to moisture. Values change depending on the coefficient used.
Together with Patients and Healthcare Professionals
- Research & Development
- Stable Supply
- Manufacturing Pharmaceuticals that are Secure, Safe, and Convenient to Use
- Information Provision
- Drug Safety / Quality Assurance
- Solving Issues Related to Improving Access to Healthcare
Together with Employees
- Human Resources Development
- Promoting Diversity & Inclusion
- Work-Style Innovation
- Occupational Health and Safety
- Health and Productivity Management
