
Management Promotion of Fair Operating Practices(Under review)
- Initiatives for Fair Business Practices
- Code of Practice
- Appropriate Promotion Activities Initiatives
- Initiatives Related to Transparency with Medical Institutions and Patient Organizations
- Initiatives to Prevent Bribery and Corruption
- Dealing with Antisocial Forces
- Protection of Intellectual Property Rights
Initiatives for Fair Business Practices
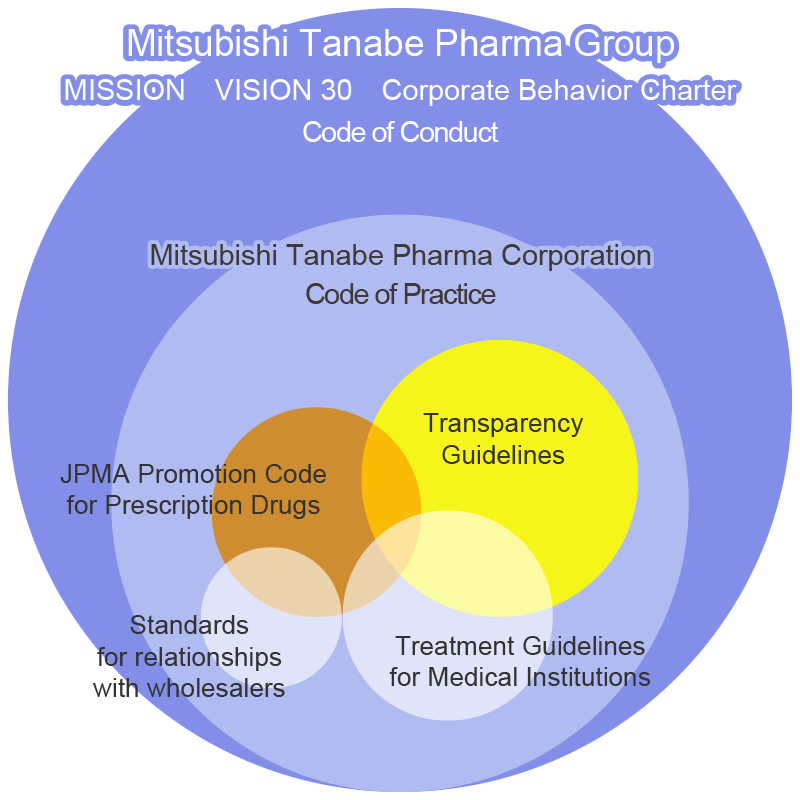
The Corporate Behavior Charter of the Mitsubishi Tanabe Pharma Group states that we will strive to maintain high ethical standards and place priority on fairness and integrity in all activities. Additionally, we formulated the Mitsubishi Tanabe Pharma Corporation Code of Practice and each activity is conducted in strict observance of the following independent standards.
- Mitsubishi Tanabe Pharma Promotion Code for Prescription Drugs
- Transparency Guidelines
- Global Policy for the Prevention of Bribery and Corruption
- Rules for Activities to Provide Sales Information on Ethical Drugs
- Treatment Guidelines for Medical Institutions
Code of Practice
The Japan Pharmaceutical Manufacturers Association (JPMA), of which Mitsubishi Tanabe Pharma is a member company, put the JPMA Code of Practice into effect in 2013. This establishes behavioral standards that must be observed by the executives and employees of the member companies in their interactions with researchers, healthcare professionals, patient organizations, wholesalers, etc. In response, the Company established and put into effect the Mitsubishi Tanabe Pharma Corporation Code of Practice (Japanese language only). All executives and employees of the Company and Group companies in Japan are required to follow this code not only in promotion endeavors designed for healthcare professionals, medical institutions, and others, but also in all other corporate activities, including testing and R&D, information provision activities for persons other than healthcare professionals, cooperation with patient organizations, and relationships with wholesalers. Overseas Group companies comply with the codes of each country based on the International Federation of Pharmaceutical Manufacturers and Associations’ Code of Practice (IFPMA Code).
- Applicable to all Company executives and employees
- Executives and employees of Group companies must also comply with this code
- Must be followed in promotion activities and all other corporate activities for healthcare professionals, medical institutions, etc.
Appropriate Promotion Activities Initiatives
Definition of Promotion
In the Japan Pharmaceutical Manufacturers Association’s Code of Practice (JPMA Code), for a pharmaceutical company, the word “promotion” as it is used here does not refer to “sales promotion.” Rather, it means “to engage with healthcare professionals in the provision, collection, and communication of drug information and promote the proper use and adoption of prescription drugs on the basis of those interactions.”
Pursuing Promotional Activities
The Promotion Code for Prescription Drugs, which is a part of the JPMA code of practice, describes details of promotions conducted by member companies. In accordance with the intent of the Promotion Code, we established the Mitsubishi Tanabe Pharma Promotion Code for Prescription Drugs to promote the appropriate use and dissemination of ethical drugs.
Additionally, in accordance with the Guidelines for Provision of Sales Information on Prescription Drugs, which sets forth a standard for appropriate sales information provision activities for ethical drugs by the Ministry of Health, Labour and Welfare, we have instituted our own Rules for Activities to Provide Sales Information on Ethical Drugs. Further, we have established the Appropriate Supervisory Committee that includes committee members from outside the company and is intended to provide advice on promotional materials and on the activities of the department that supervises the provision of sales information, and are working to promote the appropriate use of ethical drugs.
For lectures that serve as opportunities to promote ethical drugs, we use explanatory materials to provide careful explanations to presenters in advance, thus ensuring the appropriateness of lectures at the Mitsubishi Tanabe Pharma Group.
Ensuring Transparency
The aim of the Japan Fair Trade Council of the Medical Devices Industry is to restrict unjustifiable premiums, such as money, goods, services, etc., provided as an inducement to engage in transactions to ensure fair competition among businesses. The council has its legal basis in the Act against Unjustifiable Premiums and Misleading Representations.
Accordingly, we have established Treatment Guidelines for Medical Institutions to prevent the distortion of appropriate drug usage and unjustifiable customer inducements and increase the transparency of transactions by restricting the provision of unjustifiable premiums.
Supervisory System
We established the Promotion Audit Department to supervise sales information provision activities in order to promote our initiatives for proper promotion activities, and improved our systems.
Initiatives Related to Transparency with Medical Institutions and Patient Organizations
Initiatives Related to Medical Institutions
To support not only the discovery of innovative drugs but also the provision and collection of information for the purpose of appropriate drug usage, collaboration and alliances among pharmaceutical companies, universities, and medical institutions are indispensable. However, as these alliance activities become more common, there are increasing opportunities for medical institutions and healthcare professionals to be significantly involved with specific companies or products, and there could be concerns about the extent to which the judgment of both is influenced by this situation.
Formulation of guidelines
In July 2011, the Company formulated its guidelines for transparency in relationships with medical institutions. The purpose of these initiatives is to secure a broad understanding from society regarding the contribution made by the Company’s business activities to progress in medicine, pharmacology, and the other life sciences as well as the Company’s high ethical standards in its business activities.
Information disclosure
The record of payments to medical institutions by the Group has been disclosed on the Company’s website since fiscal 2012. From fiscal 2019, information has been disclosed in compliance with the Clinical Trials Act.
Regarding the provision of compensation or funds to doctors or to healthcare related institutions or organizations in Europe or the U.S., we are conducting information disclosure in an appropriate manner in accordance with guidelines and laws formulated in Europe and the U.S.
Management structure
In August 2014, the Company formulated guidelines for managing conflicts of interest with medical and research institutions. We have established principles for avoiding problems with conflicts of interest and a system for managing conflicts of interest, and we are working to operate this system in an appropriate manner.
Initiatives Related to Patient Organizations
First, it is important for corporate activities to be based on a high level of ethical standards and mutual understanding with respect for the independence of patient organizations. On that basis, to secure a broad understanding from society regarding our contribution to the activities and development of patient organizations, in April 2013, we formulated our guidelines for transparency in relationships with patient organizations. Since fiscal 2013, information regarding the funds and labor provisions and rewards to these patient organizations are disclosed on the Company’s website.
Initiatives to Prevent Bribery and Corruption
Bribery and corruption in business not only hinder proper commercial transactions, but they can also have harmful effects, such as serving as the source of funding for anti-social forces. Recently, regulations for bribery and corrupt practices are being reinforced in countries around the world.
The Group has established the “Mitsubishi Tanabe Pharma Group Global Anti-Bribery and Corruption Policy,” which applies to all Group companies, with the aim of further strengthening its approach toward prevention of bribery and corrupt practices.
The Group declared in the Policy that it will take a “zero-tolerance approach” to bribery and corrupt practices, and promised that it will not engage in bribery or corrupt practices. The Group also stated it will establish and operate an in-house system to eradicate bribery and corrupt practices.
Moreover, to further clarify the content of this policy, we formulated relevant guidelines for countries and regions where risks are thought to be particularly high, and we are implementing appropriate responses in line with the laws, regulations, and business practices of each country.
In fiscal 2023, there were no incidences of violations or sanctions related to bribery or corruption at the Mitsubishi Tanabe Pharma Group.
Dealing with Antisocial Forces
In accordance with rules for the elimination of crime syndicates, the Group’s basic policies regarding corporate extortionists, crime syndicates, and other antisocial forces are to not fear them, to not provide any funds to them, and to shun all contact with them. Therefore, we have taken the initiative to constantly gather information on antisocial forces and verify our business partners in cooperation with specialized external institutions such as the police. In the face of unreasonable demands, the Group will respond with a resolute stance that is unyielding and uncompromising. Moreover, executives and employees in their day-to-day business activities, must consistently avoid relationships with antisocial forces, adhere strictly to relevant laws and ordinances, and act in accordance with social ethics.
Protection of Intellectual Property Rights
Under its philosophy of contributing to the healthier lives of people around the world through the creation of pharmaceuticals, the Group has established an intellectual property policy as a basis for providing new medical opportunities and to protect and make effective use of its globally competitive intellectual properties.
In addition to filing, acquiring, and maintaining intellectual property rights including patents and trademarks, we promote an intellectual property strategy that is integrated with our business and R&D strategies, and work to ensure our rights not only at the initial research stage, but also at the appropriate time tailored to the product lifecycle. As a result, the Group held 838 registered patents as of March 31, 2024.
Furthermore, the Company respects third-party valid intellectual property rights by managing intellectual property risks through investigation into the rights of third parties. At the same time, we are working to create a system to protect and utilize our intellectual property by taking legal action and other measures, depending on the situation, in case a third party infringes upon our intellectual property rights.
In principle, the Group does not enforce its patent rights in the Least Developed Countries (LDCs) stipulated by the United Nations in order to contribute to healthcare access in economically deprived areas around the world.
- Respect for Human Rights
- R&D Ethics
- Compliance
- Risk Management
- Promotion of Fair Operating Practices
